Glioblastoma studies show promise
Carolina’s researchers are coming up with new therapies and technology to treat the deadly brain cancer.
Receiving a diagnosis of glioblastoma multiforme is devastating. About half of people with the aggressive brain tumor live no more than 18 months, and nearly 70% do not survive past five years. The depth and breadth of Carolina’s research on treatments are especially encouraging since the standard of care for the disease hasn’t changed since 2005.
T-cell therapy
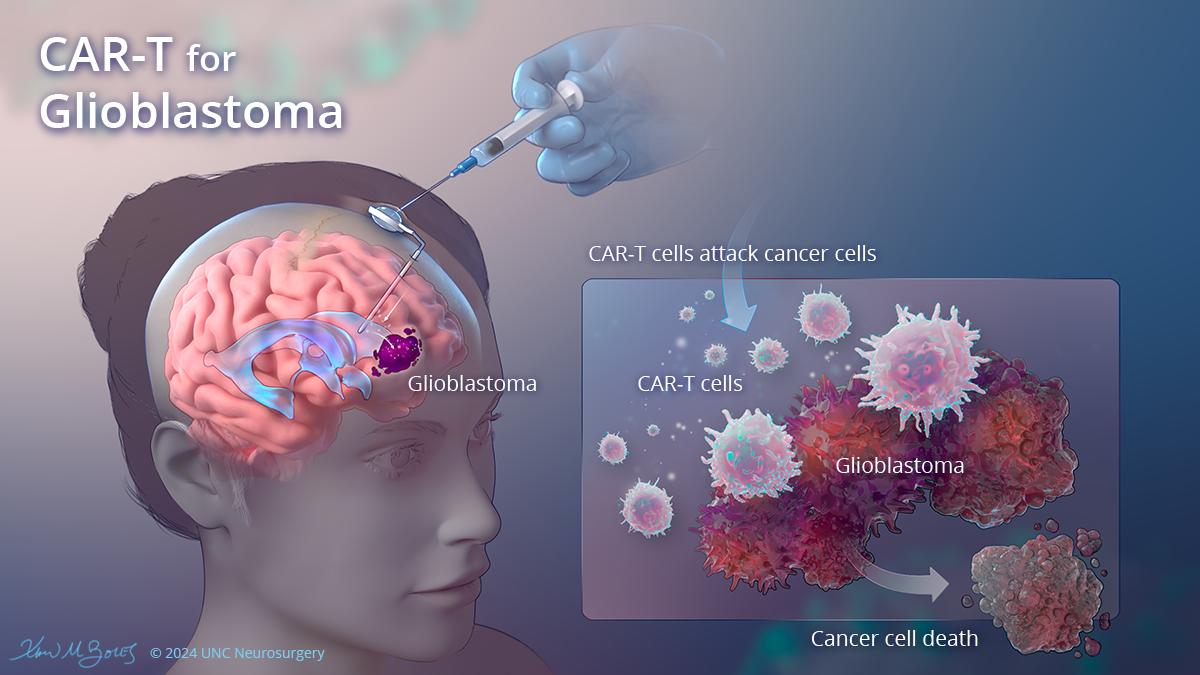
One UNC Healthclinical trialbegun in 2022 intends to evaluate the safety and patients’ toleration of chimeric antigen receptor T-cell therapy. The trial is treating patients whose cancer has not successfully responded to one or more treatments.
The CAR-T process involves extracting specific immune cells from patients, engineering the cells in a lab to identify tumor cells displaying a specific molecular target, then re-infusing the cells to fight the patient’s brain tumor. Now programmed to have “chimeric antigen receptors” or CARs, the T-cells latch onto tumor cells and destroy them.
Dr. Yasmeen Rauf, assistant professor in the UNC School of Medicine neurology and neurosurgery departments and a medical oncologist with the UNC Lineberger Comprehensive Cancer Center, leads the study.
Multicenter clinical trial
A UNC Healthmulticenter clinical trialaims to enroll93 newly diagnosed glioblastoma patients in a study to assess the safety and effectiveness of IGV-001, an immunotherapy developed by the biotechnology company Imvax Inc. UNC Health is the exclusive site for this unique trial in North Carolina and the South. Fourteen UNC Health satellite sites throughout the state will accommodate patients far from Chapel Hill. The patients will receive weekly treatments at UNC Hospitals.
The study involves implanting small biodiffusion chambers containing either IGV-001, which is designed to induce a broad and durable immune response against tumors, or a placebo after tumor-removal surgery. Six weeks after treatment, patients begin standard-of-care treatment, chemotherapy and radiotherapy.
Key researchers are Dr. Soma Sengupta, vice chair of the medical school’s neurology department and the study’s principal investigator; Dr. Dominique Higgins, assistant professor in the neurosurgery department and member of Lineberger Comprehensive Cancer Center; and Dr. Carlos David, neurosurgery professor.
Personalized immune therapy
A November 2022 study found that a newpersonalized immune therapyfrom Northwest Biotherapeutics improved survival time for many patients. Dr. Matt Ewend, Van L. Weatherspoon Jr. Eminent Distinguished Professor of Neurosurgery and Lineberger member, led the trial site at the N.C. Basnight Cancer Hospital and co-authored the study report.
‘Brain slice’ technology
At the Eshelman School of Pharmacy,associate professor Shawn Hingtgen and research assistant professor Andrew Satterlee have developeda “brain slice” toolto help physicians design better treatment plans. The tool houses the brain tumor sample and keeps it alive while researchers test different drugs to see which one is most successful in attacking the cancerous tumor.
Hintgen is associate director of the Brain Slice Technology Platform in the pharmacy school’s Eshelman Institute for Innovation, a clinical assistant professor in the medical school’s neurosurgery department and a Lineberger member. He and Satterlee said the tool helps doctors make better and quicker choices for treatment drugs and help drug development companies advance their therapeutic processes under a microscope.
Hingtgen’s team is also creating treatments by examining receptors on the surface of glioblastoma cells, such as the epidermal growth factor receptor, which regulates cell growth and division that are crucial to a cancer’s development. Using noninvasive imaging, they track changes in the receptors and the effectiveness of therapies.
Higgins is pursuing research with his surgical patients that is complementary to Hingtgen’s work and is invested in the Hingtgen team’s outcomes.